Search Results
Showing results 121 to 140 of 175

Mixtures and Solutions
Source Institutions
This activity was designed for blind learners, but all types of learners can use it to investigate heterogeneous and homogeneous mixtures and solutions, identify the differences, and explore the conce

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.
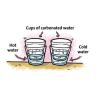
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Exploring Fabrication: Gummy Capsules
Source Institutions
In this activity, learners make self-assembled polymer spheres.

Dissolving a Substance in Different Liquids
Source Institutions
In this activity, learners make colored sugar and add it to water, alcohol, and oil to discover some interesting differences in dissolving.

Soda Geyser
Source Institutions
In this quick activity (page 1 of PDF under SciGirls Activity: Lift Off), learners will use the ever-popular soda geyser experiment to test the reactivity of the various sugar candies or mints.

Model Well
Source Institutions
In this quick activity about pollutants and groundwater (page 2 of PDF under Water Clean-up Activity), learners build a model well with a toilet paper tube.

Crash Landing!
Source Institutions
In this activity, groups cut out and sort cards showing items recovered from a crash landing on the Moon. The 12 items range from food and water to rope and matches to a self-inflating life raft.
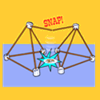
Spaghetti Bridge
Source Institutions
Play with your food while learning about engineering! Build a spaghetti bridge, then test its strength by piling on the marshmallows, raw spaghetti, raw linguine and coins.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.
Cartesian Diver
Source Institutions
In this quick activity (page 1 of the PDF under SciGirls Activity: California Fish), learners will build a simple Cartesian Diver in an empty 2-liter bottle.

The Liquid Rainbow
Source Institutions
Learners are challenged to discover the relative densities of colored liquids to create a rainbow pattern in a test tube.
Enhanced Water Taste Test
Learners conduct a "blind" taste-test of several types of enhanced or fitness water drinking water that has commercially added substances like vitamins, sugars, or herbs.

Making a Battery from a Potato
Source Institutions
In this electrochemistry activity, young learners and adult helpers create a battery from a potato to run a clock.

Crushing Test
Source Institutions
In this activity, learners design a crushing test and discover that identifying and controlling the variables may be difficult.

Frijolitos
Source Institutions
Esta actividad enseña la proporción y razón por hacer que los aprendices hagan "ensaladas" que combinan tres tipos de frijoles en tres combinaciones diferentes.

Protect That BRAIN!: Mr. Egghead
Source Institutions
This activity demonstrates the importance of wearing a helmet to protect the brain. An egg is used to symbolize a head with the shell as the skull and the inside of the egg as the brain.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.
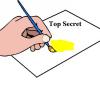
Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.
