Search Results
Showing results 161 to 180 of 385
Cooling the Mummy's Tomb
Source Institutions
In this activity, learners conduct an experiment to help Pharaoh design a better insulated tomb.

Imploding Pop Can
Source Institutions
In this dramatic activity/demonstration about phase change and condensation, learners place an aluminum can filled with about two tablespoons of water on a stove burner.

See It to Believe It: Visual Discrimination
Source Institutions
In this activity (12th on the page), learners investigate their ability to discriminate (see) different colors.

Identifying Erosion
Source Institutions
In this environmental science activity (page 3 of the PDF), leaners will identify and explain the causes of erosion.

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.

Drying It Out
Source Institutions
In this activity, learners investigate and compare the rate of drying in different conditions.

Loony Balloons
Source Institutions
In this activity, learners investigate how changing the center of gravity of a balloon affects how it travels. Learners fill a balloon with a little bit of water and insert into an empty balloon.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!
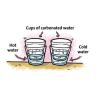
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Build A Hydrometer
Source Institutions
In this activity, learners will explore how a hydrometer works by building a working model and conducting experiments.

A Pressing Engagement
Source Institutions
In this quick and easy activity and/or demonstration, learners illustrate the effect of the weight of air over our heads.

Water Fountain
Source Institutions
In this activity, learners explore how a hydraulic pump works. Learners work in teams to design and build a unique water fountain that employs a hydraulic pump.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.
What Does Life Need to Live?
Source Institutions
In this astrobiology activity (on page 11 of the PDF), learners consider what organisms need in order to live (water, nutrients, and energy).

Which Powder is It?
Source Institutions
In this chemistry challenge, learners identify an unknown white powder by comparing it with common household powders.
