Search Results
Showing results 1 to 20 of 44

Adherence to HIV Treatment
Source Institutions
In this activity, learners simulate taking HIV antiretroviral drugs by using Tic Tac mints and Kool-Aid packets.

Mold Growth
Source Institutions
In this activity learners observe mold growth on different types of bread by measuring and recording the growth rate.

Nano Ice Cream
Source Institutions
In this activity/demo, learners discover how liquid nitrogen cools a creamy mixture at such a rapid rate that it precipitates super fine grained (nano) ice cream.
Breakfast Sweets
Source Institutions
In this math activity, learners guess which cereals contain the most sugar. Learners use the nutrition labels on the cereal boxes to find the cereal with the least amount of sugar.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.
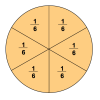
Number Sense and Computation: To Half or Half Not
Source Institutions
In this math lesson, learners use slices of bread and geoboards to explore several ways to divide a rectangle in half. Learners also identify equivalent fractions that represent one-half.

Biochemistry Happens Inside of You!
Source Institutions
In this four-part activity, learners explore how the body works and the chemistry that happens inside living things.

We all Scream for Ice Cream
Source Institutions
In this activity, learners observe how salinity affects the freezing point of water by making and enjoying ice cream.
Multi-Variable Relations: Stressed to the Breaking Point
Source Institutions
In this math lesson, learners explore the relationship between the thickness of a spaghetti bridge, the length of the bridge, and the amount of weight that can be supported by the bridge.

How Boulders Are Born
Source Institutions
In this activity, learners review and discuss weathering, erosion and mass wasting, to gain a stronger understanding of how Hickory Run’s Boulder Field was formed after the Laurentide Continental Glac

Burn a Peanut
Source Institutions
In this activity, learners burn a peanut, which produces a flame that can be used to boil away water and count the calories contained in the peanut.

Not Just A Bag Of Beans
Source Institutions
In this activity, learners count and measure kidney beans to explore natural selection and variation. Learners measure the length of 50-100 beans.

Chromatography
Source Institutions
In this chemistry activity, learners will separate a mixture of FD&C dyes (colors certified and allowed by the US for the Food, Pharmaceutical, Cosmetics & Personal Care industry) to practice

Food for the Brain
Source Institutions
In this activity, learners dissect a piece of pizza to learn about nutrients important for health.
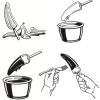
T. rex Cretaceous Treat
Source Institutions
In this activity, learners make edible T. rex teeth (with adult assistance). The treat is a white and dark chocolate covered banana on a stick.

Mix It Up
Source Institutions
In this math lesson, learners are introduced to proportional reasoning through modeling, sharing, and questioning techniques.

Observing Different Microbes
Source Institutions
In this activity, learners use a microscope to examine three different microbes: bacteria, yeast and paramecia. Educator will need to prepare the yeast solution one day before the activity.

Chemistry in the Kitchen
Source Institutions
In this kitchen chemistry activity, learners explore the chemistry of crystals by making sugar crystals, consider a common chemical reaction type responsible for the rising of muffins and cake in the
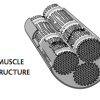
Muscle Fibers
Source Institutions
In this activity about human anatomy (page 20 of PDF), learners investigate the structure of muscles by comparing yarn and cooked meat.
Do Plants Need Light?
Source Institutions
In this food science activity, learners conduct an experiment that demonstrates the importance of light to plants.
