Search Results
Showing results 1 to 17 of 17

Surface Area
Source Institutions
In this demonstration, learners discover that nanoparticles behave differently, in part because they have a high surface area to volume ratio.

The Electric Squeeze
Source Institutions
In this activity/demo about piezoelectricity, learners discover how some crystals produce electricity when squeezed.

Making a Battery from a Potato
Source Institutions
In this electrochemistry activity, young learners and adult helpers create a battery from a potato to run a clock.

Fast Rusting
Source Institutions
In this activity, learners conduct an experiment to find out if steel wool will weigh more or less when it is burned. Learners will explore the effects of oxidation and rusting on the steel wool.

Size and Scale: Probing and Predicting
Source Institutions
In this quick activity about predicting (located on page 2 of the PDF under Where's Nano?
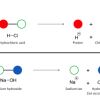
Chemical Identification
Source Institutions
In this activity, learners discover how a cabbage juice indicator helps identify acids and bases, and how iodine indicates the presence of starch.

Carbon Configurations
Source Institutions
In this activity, learners use geometry to predict the shape of carbon. Learners twist and attach chenille stem pieces that represent bonds between different carbon atoms.

Mixtures and Solutions
Source Institutions
This activity was designed for blind learners, but all types of learners can use it to investigate heterogeneous and homogeneous mixtures and solutions, identify the differences, and explore the conce

Mystery Shapes
Source Institutions
In this activity, learners describe an object they can’t see. After someone picks outs a few mystery objects and places them in a pillowcase, learners will investigate using only their hands.
Periodic Pegboard
Source Institutions
In this activity, learners use pegboard and straws to build a three-dimensional model of the periodic table.

Curie Point
Source Institutions
In this activity best suited as a demonstration, learners observe that when a piece of iron gets too hot, it loses its ability to be magnetized.

Mighty Molecules
Source Institutions
In this activity, learners use marshmallows and gum drops to construct seven models of molecules. Learners classify (solid, liquid or gas) and draw diagrams of the molecules.

Gas Model
Source Institutions
This highly visual model demonstrates the atomic theory of matter which states that a gas is made up of tiny particles of atoms that are in constant motion, smashing into each other.

Big Things Come in Little Packages
Source Institutions
As a group, learners investigate three packages which are all the same size and shape, but have different contents. One is filled with foam, one is filled with wood, and one is filled with metal.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

Balanced Budget Chemistry
Source Institutions
In this activity, learners balance chemical equations and discover the law of conservation of mass. Learners use coins to model molecules to balance the equations.

Exploring the Nanoworld with LEGO Bricks: Structures at the Nanoscale
Source Institutions
In this activity (pages 7-16), learners model various crystal structures with LEGOs. This activity also contains additional links that explain how to create other crystal structures.
