Search Results
Showing results 1 to 15 of 15

Matter on the Move
Source Institutions
Learners observe and conduct experiments demonstrating the different properties of hot and cold materials.

Separation Anxiety
Source Institutions
In this activity, learners discover the primary physical properties used to separate pure substances from mixtures.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.

Why Circulate?
Source Institutions
In this activity related to the human circulatory system (on page 10 of the PDF), learners observe the dispersion of a drop of food coloring in water, draw conclusions about the movement of dissolved

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

Biochemistry Happens Inside of You!
Source Institutions
In this four-part activity, learners explore how the body works and the chemistry that happens inside living things.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.

Using Color to See How Liquids Combine
Source Institutions
Learners add different liquids (water, salt water, alcohol, and detergent solution) to water and observe the different ways the different liquids combine with water.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Crystal Painting
Source Institutions
In this activity, learners will "paint" their own crystal artwork by creating a picture with a super saturated salt solution.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.
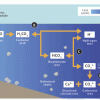
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.
