Search Results
Showing results 1 to 20 of 38

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.

Exploring Baking Powder
Source Institutions
In this activity, learners examine baking powder, a combination of three powders: baking soda, cream of tartar, and cornstarch.

Formation of a Precipitate
Source Institutions
Learners create hard water by mixing Epsom salt and water. Then they compare what happens when soap solution is mixed with hard water and regular water.

Hot and Cold
Source Institutions
In this chemistry challenge, learners discover that many chemical reactions involve heat loss or gain.

Which Powder is It?
Source Institutions
In this chemistry challenge, learners identify an unknown white powder by comparing it with common household powders.

Finding Colors
Source Institutions
In this chemistry challenge, learners combine acids and bases in a universal indicator to create five different colors.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.

Invisible Ink
Source Institutions
In this simple chemistry activity (page 1 of PDF under SciGirls Activity: Colorblind Dogs) about acids and bases, learners will mix a baking soda and water solution and use it to paint a message on a

Neutralizing Acids and Bases
Source Institutions
Learners use their knowledge of color changes with red cabbage indicator to neutralize an acidic solution with a base and then neutralize a basic solution with an acid.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Color Changes with Acids and Bases
Source Institutions
Learners mix a variety of substances with red cabbage juice. The juice changes color to indicate whether each substance is an acid or a base.

Red, White and Blue I Demonstration
Source Institutions
In this chemistry demonstration, learners observe a chemical reaction that produces a colorful effect.

Using Chemical Change to Identify an Unknown
Source Institutions
In this activity, learners will develop a method to test five similar-looking powders (baking soda, baking powder, cream of tartar, detergent, and cornstarch) with four test liquids (water, vinegar, i

Animal Scent
Source Institutions
This activity (on page 3 of the PDF under GPS: Animal Scent Activity) is a full inquiry investigation into animal behavior.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.

Finding Red
Source Institutions
In this chemistry challenge, learners systematically investigate which combination of four solutions produces a deep red color.

Fizzy Fun
Source Institutions
In this activity, learners test what happens when they put baking power on different frozen liquids.
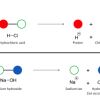
Chemical Identification
Source Institutions
In this activity, learners discover how a cabbage juice indicator helps identify acids and bases, and how iodine indicates the presence of starch.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.
