Search Results
Showing results 21 to 40 of 62
Sodium Acetate Hand Warmers
Source Institutions
In this activity, sodium acetate hand warmers are used to introduce learners to supersaturated solutions, crystallization, and exothermic reactions.

How Greenhouse Gases Absorb Heat
Source Institutions
Learners observe two model atmospheres -- one with normal atmospheric composition and another with an elevated concentration of carbon dioxide.
The Return of El Nino
Source Institutions
In this activity related to climate change and data analysis, learners examine temperature and precipitation data to determine if climate variations are due to El Niño.

Our Solar System to Scale
Source Institutions
In this activity, learners plan and create a 24-foot long, two-dimensional model of our solar system, and compare and contrast the differences between planets and the sun.

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!
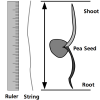
Easy PEAsy Seed Germination
Source Institutions
In this activity, learners determine the necessary conditions for pea seed germination.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Let's Dew It!
Source Institutions
From the Weather Watchers featured theme on the CYBERCHASE website. Learners will conduct experiments to discover how air temperature and humidity work together to make condensation, dew, and fog.

Make a Terrarium
Source Institutions
In this activity, learners make a miniature greenhouse or "terrarium" to explore the greenhouse effect.
Effects of Solar Radiation on Land and Sea
Source Institutions
In this activity, learners explore the different heating properties of soil and water.

Try Your Hand at Nano
Source Institutions
This lesson focuses on two simple activities that younger learners can do to gain an appreciation of nanotechnology. First, learners measure their hands in nanometers.

Heat Capacity: Can't Take the Heat?
Source Institutions
Why is ocean water sometimes the warmest when the average daily air temperature starts to drop? In this activity, learners explore the differing heat capacities of water and air using real data.

Saguaro Nest Cavities
Source Institutions
This activity (on page 3 of the PDF under GPS: Cactus Activity) is a full inquiry investigation into how some desert birds keep their cool.

Temperature Affects Dissolving
Source Institutions
Learners design their own experiment to compare how well cocoa mix dissolves in cold and hot water. They will see that cocoa mix dissolves much better in hot water. Adult supervision recommended.

Lift Off!
Source Institutions
This activity (on page 2 of the PDF under SciGirls Activity: Lift Off) is a full inquiry investigation into the engineering challenges of sending scientific sensors into space.

Moldy Jell-O
Source Institutions
In this laboratory activity, learners design an experiment to evaluate how environmental factors influence the growth of molds.

Canned Heat
Source Institutions
In this activity, learners explore how light and dark colored objects absorb the Sun's radiations at different rates.

Gas Model
Source Institutions
This highly visual model demonstrates the atomic theory of matter which states that a gas is made up of tiny particles of atoms that are in constant motion, smashing into each other.
The Daily Ups and Downs
Source Institutions
In this activity, learners graph 48 hourly air temperatures from a local weather observation site and observe the diurnal temperature variations.

Temperature vs. Height: Soda Geyser Series #6
Source Institutions
In this activity, learners conduct a controlled experiment to examine how temperature will affect the height of a soda geyser.
