Search Results
Showing results 301 to 320 of 948
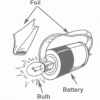
Bright Lights
Source Institutions
In this activity about electricity, learners imagine that they are out in the wilderness and it is getting dark. Their task is to use the materials supplied to build a simple flashlight.

What is a Nanometer?
Source Institutions
This lesson focuses on how to measure at the nanoscale and provides learners with an understanding how small a nanometer really is.

Musical Gloves
Source Institutions
Put on a pair of gloves and be the conductor of your invisible orchestra!

Balls and Ramps
Source Institutions
In this activity, learners use simple, everyday materials to experiment with balls and ramps.
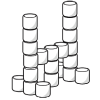
Marshmallow Models
Source Institutions
No glue is needed for learners of any age to become marshmallow architects or engineers.

Hot Stuff!: Testing Ice
In this demonstration, learners compare and contrast regular water ice to dry ice (frozen carbon dioxide). Both samples are placed in a solution of acid-base indicator.

Hot Cans and Cold Cans
Source Institutions
Learners apply their knowledge of heat transfer to design two cans - one that will retain heat and one that will cool down quickly.

How Greenhouse Gases Absorb Heat
Source Institutions
Learners observe two model atmospheres -- one with normal atmospheric composition and another with an elevated concentration of carbon dioxide.
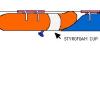
Balloon Staging
Source Institutions
In this activity, learners simulate a multistage rocket launch using party balloons, fishing line, straws, and a plastic cup.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.

Bend That Bar
Learners play the role of materials engineers as they test the flexibility of different materials.

How the Rubber Meets the Road
Source Institutions
In this activity, learners explore how engineers design tire treads to increase safety and reliability.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Homemade Rube Goldberg Machine
Source Institutions
In this fun and, at times, hilarious force and motion activity, learners will use household objects to build a crazy contraption and see how far they can get a tennis ball to move.

Carbon Configurations
Source Institutions
In this activity, learners use geometry to predict the shape of carbon. Learners twist and attach chenille stem pieces that represent bonds between different carbon atoms.

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!

Make a Mobile!
Source Institutions
In this activity, learners make mobiles to explore the concepts of balance, counterbalance, weight, and counterweight.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Hang Time
Source Institutions
In this physics activity, learners will build their own parachutes out of tissue paper. They will explore the effects of weight, height, and design on the parachutes' speed and stability.
Finding the Size of the Sun and Moon
Source Institutions
In this activity, learners build a simple pinhole viewer. They use this apparatus to project images from a variety of light sources, including a candle, the Sun, and the Moon.
