Search Results
Showing results 1 to 20 of 34
Pour Some: Measure Serving Size
Source Institutions
Make snack time into measuring time and learn to read Nutrition Facts labels. Try this when you’re using “pourable” foods, such as cereal, yoghurt, or juice.

Try Growing Your Own Mold
Source Institutions
This is a hands-on activity that uses bread and household materials to grow mold. Learners collect dust from a room, wipe it on food, and contain it. One to seven days later, mold has grown.
Chemical Reactions in Your Mouth
Source Institutions
In this chemistry activity (page 5 of the PDF), learners will see that chewing is more than just the crushing up of food; there is actually a chemical change going on at the same time.

Static Shock!
Source Institutions
In this hands-on activity, learners explore static electricity through the use of common household products. They also explore the connection between static electricity and cold weather.

Egg-stra Strength
Source Institutions
In this physics activity, learners will investigate the strength of egg shells.
Growing Rock Candy
Source Institutions
In this activity, learners make their own rock candy. Crystals will grow from a piece of string hanging in a cup of sugar water. The edible crystals may take up to a week to form.

Rock Candy
Source Institutions
In this yummy chemistry activity which requires adult supervision, learners use sugar and water to explore how crystals form.
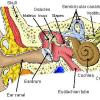
Model Eardrum
Source Institutions
In this activity (last activity on the page), learners make a model of the eardrum (also called the "tympanic membrane") and see how sound travels through the air.
Marshmallow Models
Source Institutions
No glue is needed for learners of any age to become marshmallow architects or engineers.

Exploring Baking Powder
Source Institutions
In this activity, learners examine baking powder, a combination of three powders: baking soda, cream of tartar, and cornstarch.

One In The Hand
Source Institutions
In this physics demonstration, learners are challenged to break a raw egg just by squeezing it. Learners will be shocked by their inability to complete the deceivingly simple challenge.
Why is the Sky Blue?
Source Institutions
In this activity, learners create a "mini sky" in a glass of water in a dark room.

Neutralizing Acids and Bases
Source Institutions
Learners use their knowledge of color changes with red cabbage indicator to neutralize an acidic solution with a base and then neutralize a basic solution with an acid.

Root Beer Float
Source Institutions
In this quick activity/demonstration about density, learners examine what happens when two cans of root beer--one diet and one regular--are placed in a large container of water.

Soap: Sometimes oil and water do mix!
Source Institutions
In this activity (on page 2 of PDF), learners mix oil and water. Then, they add soap and observe what changes! The activity demonstrates how oil and water don't mix, except when soap is added.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.

Mysterious M&M's
Source Institutions
Learners place an M&M candy in water and observe what happens. The sugar-and-color coating dissolves and spreads out in a circular pattern around the M&M.

Color Changes with Acids and Bases
Source Institutions
Learners mix a variety of substances with red cabbage juice. The juice changes color to indicate whether each substance is an acid or a base.

Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.
