Search Results
Showing results 321 to 340 of 507

Joe's Place
Source Institutions
In this math activity (Page 8 of the Dining Out! PDF), younger learners select items from a menu and count out the total amount needed using the fewest bills and coins possible.

The Thousand-Yard Model
Source Institutions
This is a classic exercise for visualizing the scale of the Solar System.

Solar Convection
Source Institutions
In this activity, learners add food coloring to hot and cold water in order to see how fluids at different temperatures move around in convection currents.
Making Regolith
Source Institutions
This lesson will helps learners answer the question: How does the bombardment of micrometeoroids make regolith on the moon?
How Does Water Climb a Tree?
Source Institutions
In this activity, learners conduct an experiment to explore how water flows up from a tree's roots to its leafy crown.

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.

What's in the Water?: Biotic and Abiotic Elements in Aquatic Ecosystems
Source Institutions
In this investigation learners explore the differences between, and interdependence of, living and nonliving elements in a water ecosystem.

Wheat Evolution: Dough Rising and Baking
Source Institutions
In this activity (Page 25 of PDF), learners investigate the evolution of wheat by creating dough from different flours, observing the samples of dough as they rise, and then baking the dough.

Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.
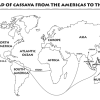
Green Travelers
Source Institutions
In this activity (on pages 23-29), partners use the Plant Traveler Cards, along with a world map and map worksheets, to follow plants such as cassava, chocolate and coffee that grew first in one part

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.

Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Exploring Fabrication: Gummy Capsules
Source Institutions
In this activity, learners make self-assembled polymer spheres.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.
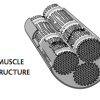
Muscle Fibers
Source Institutions
In this activity about human anatomy (page 20 of PDF), learners investigate the structure of muscles by comparing yarn and cooked meat.

Scent Tag
Source Institutions
In this matchmaking activity (on page 2 of the PDF under GPS: Animal Scent Activity), learners will each have a scented cotton ball taped to their shoulder. The scent (e.g.

Fungus Among Us
Source Institutions
In this environmental health activity, learners grow and observe bread mold and other kinds of common fungi over the course of 3-7 days.
