Search Results
Showing results 1 to 20 of 119

Say Cheese!
Source Institutions
Create a chemical reaction that makes cheese! This hands-on activity demonstrates that molecules and atoms are tiny particles that make up everything around us.

Wiggly Water
Source Institutions
This is a simple and fun activity for learners to explore water and colors.
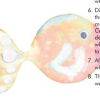
Universal Indicator Rainbow Trout
Source Institutions
In this activity, learners cut out a fish and then "paint" it using universal indicator and acids and bases.

Soap: Sometimes oil and water do mix!
Source Institutions
In this activity (on page 2 of PDF), learners mix oil and water. Then, they add soap and observe what changes! The activity demonstrates how oil and water don't mix, except when soap is added.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Exploring Materials: Liquid Crystals
Source Institutions
In this activity, learners discover that the way a material behaves on the macroscale is affected by its structure on the nanoscale.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.
Separating with Chromatography
Source Institutions
In this experiment, learners separate different types of molecules in marker inks (using a technique called "thin layer chromatography").

Gravity Fail
Source Institutions
In this activity, learners try pouring water out of a regular cup and a miniature cup. It’s harder than it sounds! Learners discover that different forces dominate at different size scales.

Exploring How Liquids Behave
Source Institutions
Learners apply their knowledge from a previous study to identify different liquids--water, corn syrup, and vegetable oil.

Hot and Cold
Source Institutions
In this chemistry challenge, learners discover that many chemical reactions involve heat loss or gain.

Nano Ice Cream
Source Institutions
In this activity/demo, learners discover how liquid nitrogen cools a creamy mixture at such a rapid rate that it precipitates super fine grained (nano) ice cream.

The Liquid Rainbow
Source Institutions
Learners are challenged to discover the relative densities of colored liquids to create a rainbow pattern in a test tube.

Gummy Shapes
Source Institutions
In this activity, learners use chemistry to “self-assemble” gummy shapes. Learners discover that self-assembly is a process by which molecules and cells form themselves into functional structures.

Which Powder is It?
Source Institutions
In this chemistry challenge, learners identify an unknown white powder by comparing it with common household powders.

Plant Power
Source Institutions
In this chemistry challenge, learners identify which plants have the enzyme "catalase" that breaks hydrogen peroxide into water and oxygen.

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

Making a Battery from a Potato
Source Institutions
In this electrochemistry activity, young learners and adult helpers create a battery from a potato to run a clock.

Silly Putty Investigation
Source Institutions
In this activity (located on page 7 of PDF), learners explore how Silly Putty was first invented and then attempt to make a batch of their own.

Secret Message
Source Institutions
In this activity, learners explore acid and bases as they create their own invisible ink out of baking soda and grape juice.
